(NEW YORK) — The Centers for Disease Control and Prevention — alongside the U.S. Food and Drug Administration and local and state health departments — is continuing to investigate after 19 people across nine states have experienced harmful reactions after being given botulinum toxin injections.
The injections were either counterfeit or administered by unlicensed or untrained individuals and/or in non-health care settings, including homes or spas, according to the CDC.
On Tuesday, the FDA issued an alert to health care professionals and consumers that “unsafe counterfeit versions of Botox” were found in multiple states, warning that bogus or mishandled Botox products can lead to serious complications.
What is Botox?
Botulinum toxin is a neurotoxic protein made from a toxin produced by the bacterium Clostridium botulinum.
It blocks chemical signals from the nerves that cause muscles to contract. The muscles temporarily relax, reducing the appearance of wrinkles. Botox is the most common brand name of the injection.
Botox is most often used on the face to reduce forehead lines, frown lines and crow’s feet but can also be used for people with excessive underarm sweating, muscle disorders such as cerebral palsy, neck spasms, overactive bladder, migraines and other conditions.
Why can Botox injections be dangerous?
Forms of purified botulinum toxin are approved by the FDA for certain medical and cosmetic treatments, according to the Mayo Clinic.
They are considered safe when administered by licensed health care providers who meet medical control standards as dictated by the FDA.
However, when Botox is not correctly prepared, stored or administered, it can lead to a rare but serious illness called botulism, which is when the toxin attacks the body’s nerves. This can cause muscle paralysis, difficulty breathing and even death.
Botulism is treated with an antitoxin, which prevents the toxin from causing any more harm, according to the CDC. Fewer than five of every 100 people with botulism die, the agency says.
But even with antitoxins and medical care, people may suffer from fatigue or shortness of breath for years after the infection has cleared.
What to know about the latest series of illnesses?
So far, 19 people across nine states — including Colorado, Florida, Illinois, Kentucky, Nebraska, New Jersey, New York, Tennessee and Washington — have reported harmful reactions, according to the CDC.
The patients — all of them female — received the injections from unlicensed or untrained individuals or in non-health care settings.
The patients’ ages ranged from 25 to 59 years and the overwhelming majority, 95%, reported receiving injections for cosmetic purposes.
Patients said they experienced a variety of symptoms including blurry vision, double vision, drooping eyelids, dry mouth, difficulty sweating, slurred speech, fatigue, weakness sand difficulty breathing.
Nine were hospitalized and four were treated with botulism antitoxin because of concerns the botulinum toxin could have spread beyond the injection site.
According to the CDC, five patients were tested for botulism. The results were negative.
If you are considering botulinum toxin injections
If you are considering getting Botox, the CDC recommends asking the provider if they are licensed and trained to administer the injection. Some states have tools that allow patients to look up if the provider or setting has the appropriate license.
Additionally, patients should ask if the product has been approved by the FDA and if it has been obtained from a reliable source.
If there is any doubt about the provider, the setting or the product, the CDC advises against the injection.
“Botox injections are a medical procedure and should only be performed in a medical office by board-certified dermatologists or an appropriately trained non-physician clinician, under the direct on-site supervision of a board-certified dermatologist,” according to a statement from the American Academy of Dermatology.
If you receive the injection and are experiencing any symptoms of botulism, the CDC says to see your health care provider or to go to the emergency room immediately.
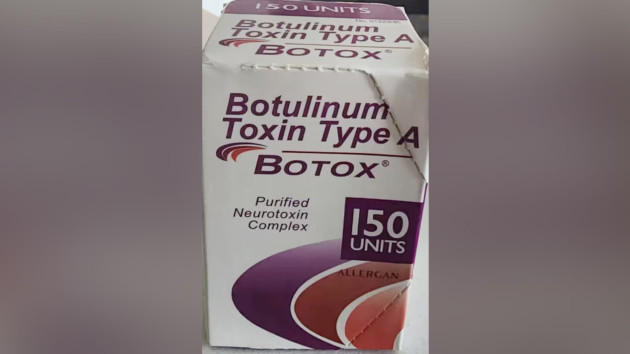
How to tell if a Botox product is counterfeit
AbbVie and Allergan — which is part of AbbVie — are the only approved Botox manufacturers and there is currently no evidence the harmful reactions are linked to the genuine product, according to the FDA.
There are some signs that a version may be counterfeit, including the outer carton and vial containing lot number C3709C3 as well as the outer carton displaying the active ingredient as “Botulinum Toxin Type A” instead of “OnabotulinumtoxinA.”
If the outer carton and vial indicates 150-unit doses, this is counterfeit because it is not a unit made by AbbVie or Allergan. Genuine products come in 50-, 100- and 200-unit doses.
Additionally, outer cartons that contain a language that is not English are counterfeit.
People who suspect counterfeit Botox products are encouraged to file a report with the FDA.
Copyright © 2024, ABC Audio. All rights reserved.